What’s in the treatment pipeline

Attending the NSPKU conference is always a mix of hope and cautious excitement, particularly when researchers and clinicians present new treatment pathways. So there was no doubt which NSPKU Conference 2025 session I’d write up first!
Prof. MacDonald laid out developments that could mean real change for everyone with PKU. In this post, I’m bringing you my notes and reflections to help make sense of where treatments might head next.
Egoo Machine & Point-of-Care (PoC) Testing
Imagine testing your Phe levels at home — no lab, no waiting! It has been the dream of many with PKU for years. There is at least one commercial machine that is awaiting approval. But, at-home phe readers are not cheap! While challenges remain in obtaining consistently accurate readings, ongoing advancements continue to improve reliability in real-world settings
Professor Anita and her team tested the Egoo Machine in her clinic, including driving to patient’s homes to get accurate data.
While practical challenges persist, such as air bubbles in pipettes affecting measurement accuracy and complexities in sample handling the underlying concept remains highly promising.
Reliable home testing has the potential to transform daily management of PKU by providing immediate feedback and reassurance.
Sepiapterin (Sephience) — a new oral BH₄ approach
This is a new treatment, different to the sapropterin (Kuvan) which is the only PKU drug treatment currently available on the NHS.Sepiapterin (Sephience) is another PKU treatment which is available elsewhere but not on the NHS.
Both sepiapterin and saptropterin aim to increase BH4 in the body, which helps to boost the phenylalanine hydroxylate enzyme (PAH) to process phenylalanine (phe). Sapropterin is a form of BH4 itself. Sepiapterin is a building block of BH4, which helps the body to make its own BH4.
This is why both are enzyme cofactors (helping to boost the PAH), but also why they work differently in different people.
Key takeaways about sepiapterin:
- Roughly twice as effective as sapropterin in certain cases
- It can increase dietary phe allowance for those already on sapropterin
- Excitingly, it seems to work for 60% of people with PKU (sapropterin only works for about 30%)
- Regulatory application expected around late 2026, though real-world access will take longer
This gives hope, particularly for those who do not respond to sapropterin, who’ve long felt left behind in PKU therapeutic development.
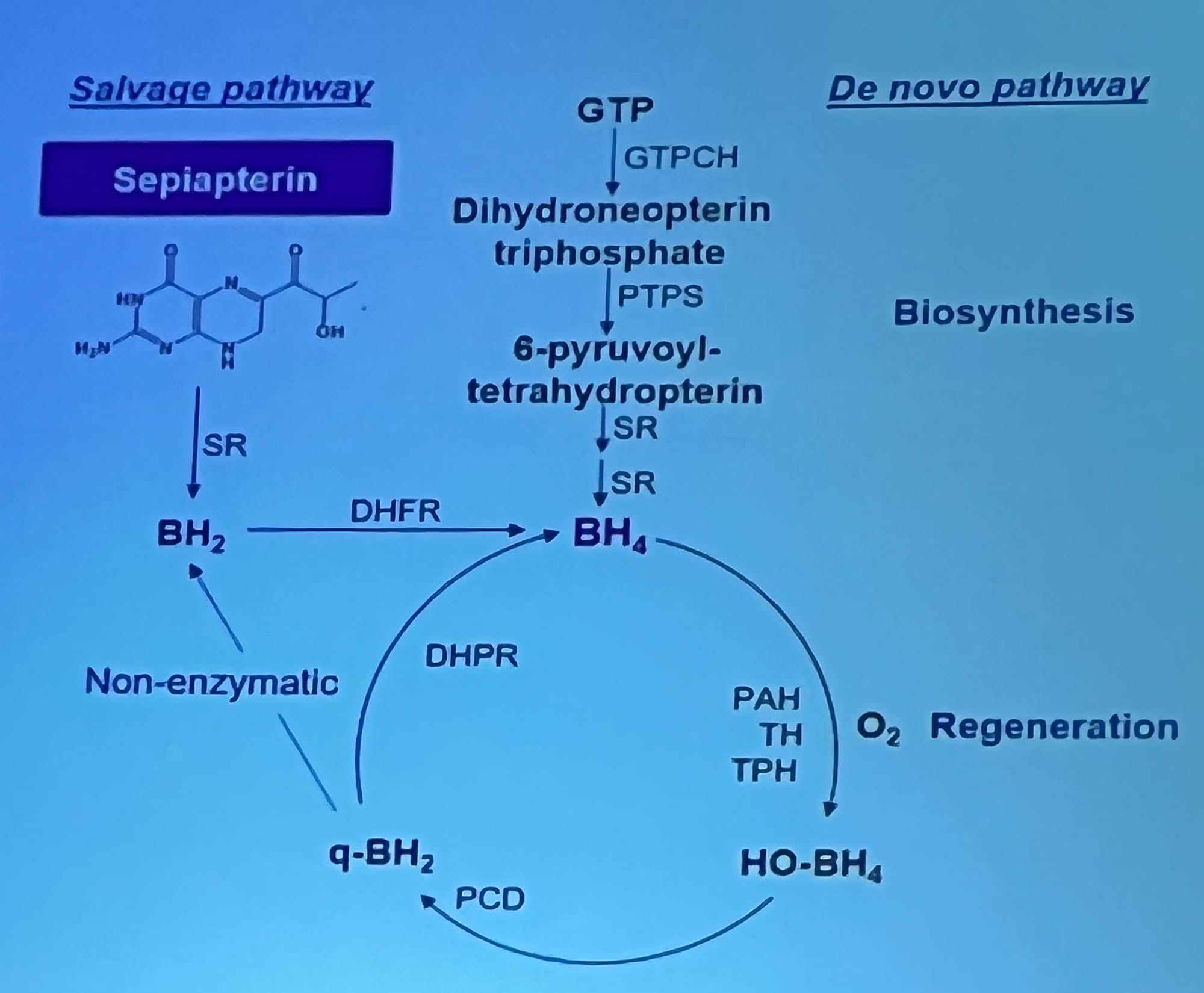
Above: One of the most striking slides showed the “salvage pathway” and “de novo pathway”, illustrating how Sepiapterin feeds into the BH₄ regeneration cycle — providing another route for phenylalanine (Phe) breakdown.
Getting kidneys involved
Professor Anita discussed two other exciting drugs, both of which work via the kidneys. Currently, the kidneys reabsorb 95% of the excess phe in the blood of someone with PKU.
The idea behind these drugs is to prevent the kidneys from reabsorbing this excess phe. Basically, instead of the excess phe going back into the blood as it does now, these drugs mean the kidneys will remove it in the urine.
JNT-517 and MZE782
Not inspiring drug names so far, but the treatments by Otsuka (former) and Maze Therapeutics (latter) are exciting.
What we know so far:
- Works across all PKU types, independent of genotype
- A massive increase in phe removed from the body in urine
- Phase 3 trials for JNT-517 are underway globallyThe UK are expected to take part in an adolescent trial next year.
- Researchers are in discussions with Maze therapeutics about Phase II trials soon.
Reflections — What this means for us
After this talk, I'm left feeling a cautious optimism. None of these treatments are quick fixes, and access often trails discovery, but every step forward matters.
A few thoughts I’m left with:
- Access matters — Sepiapterin’s data looks excellent, but until it’s approved and funded, it remains out of reach.
- Combination therapies could define the next era — imagine pairing Sepiapterin with a drug like JNT-517.
- Ease of use counts — Egoo’s challenges show that even great science must work in everyday hands.
- Advocacy is crucial — Knowing about trials is one thing; pushing for UK inclusion and NHS uptake is another.
Closing Thoughts
I’ll be following these developments closely — particularly when UK trials begin.
For anyone living with PKU, these emerging treatments represent not just medical innovation, but the possibility of more flexibility, less restriction, and maybe even a little more normality.
Do these developments make you excited, or are you worried about how changes might affect you? Please share your thoughts.
Member discussion